Prof. Dr. Raffaella Santoro
Research interests
Epigenetics and chromatin dynamics in cancer
Our group aims to understand fundamental principles of gene regulation.
We are interested in understanding how alterations of epigenetic and chromatin regulatory systems contribute to the susceptibility and development of complex diseases.
We study mechanisms of gene expression at multiple levels, from the local action of epigenetic regulatory factors and long non-coding RNAs to the regulation of genome architecture. We use a combination of molecular biology, cell biology and biochemistry methods combined with epigenomic and transcriptomic high-throughput analyses to unravel the mechanisms underlying stem cell pluripotency, differentiation, reprogramming and cancer initiation/progression and to understand epigenetic regulatory processes in physiological and pathological conditions.
Current research themes
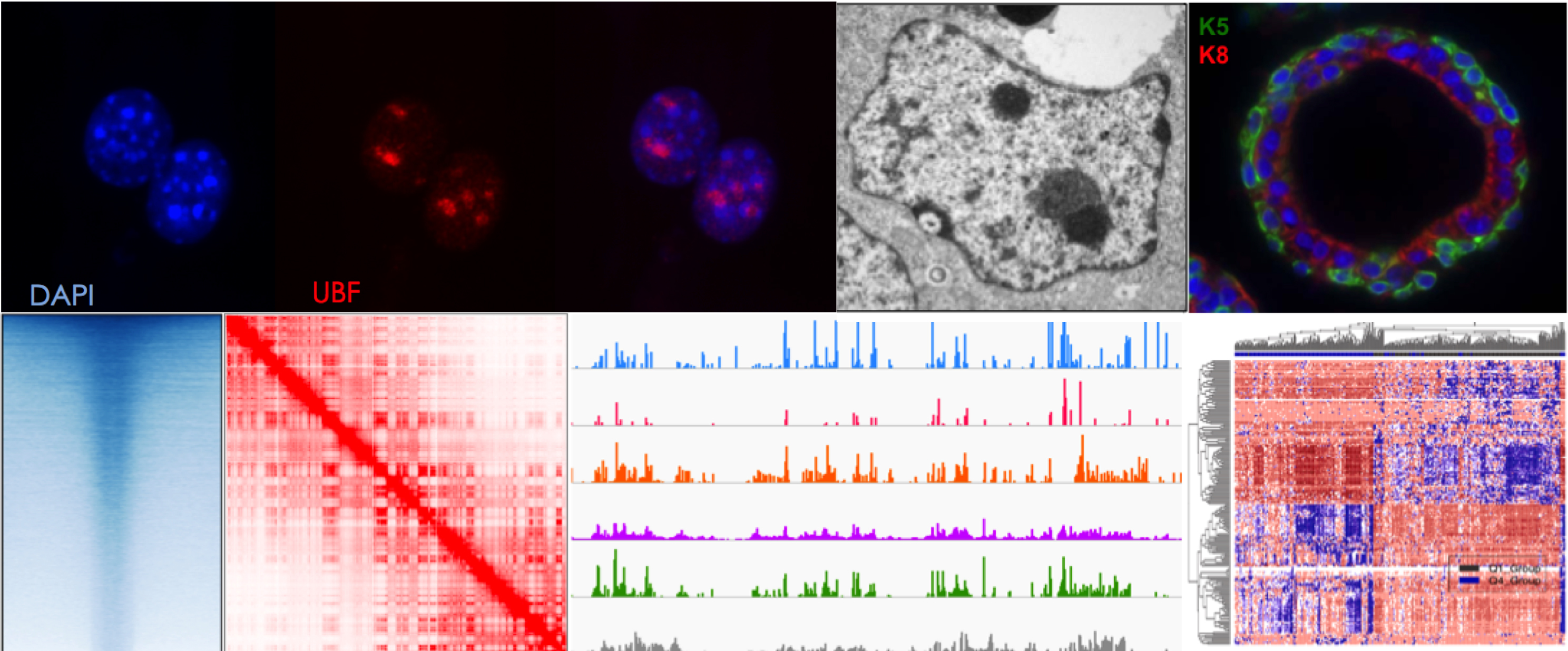
Genome organization in and around the nucleolus
The nucleolus is the most prominent substructure in the nucleus compartment, where transcription of hundreds of ribosomal RNA (rRNA) genes, rRNA processing, and ribosome subunit assembly takes place. The nucleolus is a membrane-less structure, which forms around regions of chromosomes containing stretches of rRNA gene repeats, known as nucleolar organizer regions (NORs). Transcription of rRNA genes is highly regulated through cell cycle and during cell differentiation and associated with accurate cell growth and proliferation. Furthermore, the chromatin state of rRNA genes together with the structure of the nucleolus is highly linked with the organization of the genome outside the nucleolus.
Our research aims to understand how chromatin and epigenetic mechanisms regulate rRNA genes and the role of the nucleolus in the regulation of genome organization and cell state.
Chromatin and epigenetic mechanisms in early development
Our research aims to elucidate how chromatin and epigenetic regulation is modulated during the early phase of development. We used different model systems to study how chromatin state and genome architecture are regulated during differentiation and the role of these processes in cell fate determination.
Epigenetic mechanisms in cancer
We are interested in understanding how alterations of epigenetic and chromatin regulatory systems contribute to the susceptibility and development of complex diseases.
A major focus of the lab is the elucidation of epigenetic alterations in prostate cancer. The clinical behavior of a localized prostate cancer is highly variable with some men having indolent features that can be monitored without therapeutic intervention, or highly aggressive disease that requires intervention. The clinical heterogeneity of prostate cancer is underpinned by a heterogeneous molecular landscape, which posits one of the most confounding and complex factors underlying its diagnosis, prognosis, and treatment.
Our research aims to generate models of prostate cancer to define the contribution of epigenetic alterations in disease state that will serve for the discovery of prognostic markers of disease outcome, predictive biomarkers for drug response and targets for efficient therapy.
Open positions
Postdoctoral fellow
Although there might be no immediate openings advertised here at this time, I am always interested in excellent applications from postdoctoral candidates who are keen to succeed in a competitive academic environment. Please send your documents (motivation letter, an outline of the scientific question you would like to address, CV, publication list) directly to raffaella.santoro@dmmd.uzh.ch
PhD students
We recruit PhD students through the Life Science Zurich Graduate School, which offers various competitive graduate courses. Applications can be submitted twice a year using the official website. If you are interested in joining our group for a PhD position, send your documents (motivation letter, CV, publication list and research interests) directly to raffaella.santoro@dmmd.uzh.ch
Master/Bachelor students
We have numerous research possibilities for UZH and ETH students to join our group. Please contact us directly.