Important Mechanism of Epigenetic Gene Regulation Identified
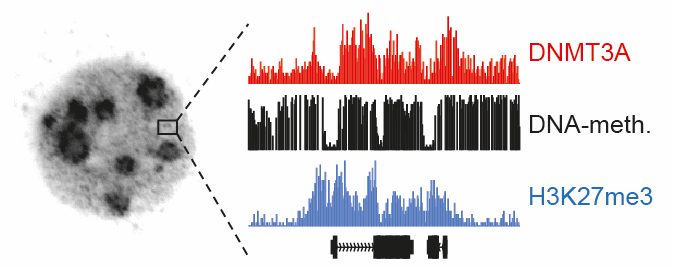
How can defective gene activity, which can ultimately lead to cancer, be avoided? Researchers at the University of Zurich have now identified a mechanism how cells pass on the regulation of genetic information through epigenetic modifications. These insights open the door to new approaches for future cancer treatments.
DNA contains the blueprint of an entire organism. Based on the information in this blueprint, every cell knows what it must become and what function it must perform. Throughout the entire lifespan of an organism, the genetic information has to be read correctly to ensure that genes are active at the right time and in the right cells. If these processes are defective, cells acquire the wrong identity – which can ultimately lead to cancer.
However, the program that determines which genes are switched on or off as a cell develops does not depend solely on DNA, but is also determined by epigenetic marks. Methylation marks on DNA act as a molecular switch that regulate gene activity in order to coordinate the cell’s specialization within the organism. How this DNA methylation is faithfully regulated, and how it can become defective, has not yet been fully resolved. However, the consequences are well-known: In many cancer types, the methylation is deposited in the wrong place. This leads to genes being read incorrectly.
Dual-layer epigenetic gene regulation
Scientists at the University of Zurich have now found new processes that regulate DNA methylation. Tuncay Baubec, professor at the Department of Molecular Mechanisms of Disease at the University of Zurich, and his team have shown that one particular protein plays an important part in this process: The DNA methyltransferase 3A (DNMT3A) enzyme is responsible for positioning the methylation to the right place on the DNA. “DNMT3A places itself preferably in close vicinity to genes that play an important role for development and makes sure that the DNA methylation around these genes is maintained,” explains Massimiliano Manzo, lead author of the study. “The DNA methylation around these genes works like a container that ensures that H3K27me3, another epigenetic modification, which normally regulates these genes, is positioned correctly.” This means that these essential genes are regulated by two epigenetic layers.
Increasing understanding of how cancer develops
The study’s findings provide important basic insights for cancer research. DNMT3A is among the most frequently mutated genes in an aggressive type of leukemia, and it plays a significant role in how this disease develops. “Our findings point toward a previously unknown function of the DNMT3A protein in the interaction of these two epigenetic modifications that are normally not directly linked. We hope that these new insights will allow us to increase our understanding of the molecular mechanisms that result in cancer and to more effectively treat this aggressive type of leukemia.” explains Professor Baubec.
Literature:
Massimiliano Manzo, Joël Wirz, Christina Ambrosi, Rodrigo Villaseñor, Bernd Roschitzki and Tuncay Baubec. Isoform-specific localization of DNMT3A regulates DNA methylation fidelity at bivalent CpG islands. Published online on 26 October 2017, EMBO Journal. DOI: 10.15252/embj.201797038