SNF Prof. Dr. Tuncay Baubec
Research interests
Systems Biology of Gene Regulation
What defines the identity of a cell? How is the same genetic code used to build more than 200 different cell types with distinct physiological and morphological properties? These fundamental questions drive our enthusiasm for understanding how information processing is regulated at the level of chromatin modifications and DNA sequence.
A multitude of DNA sequence-dependent and -independent interactions coordinate the spatiotemporal recruitment of transcriptional regulators to the genome and are required for tissue-specific gene expression and cell identity. Interference or lack of specificity in this process results in defective embryonic development and can give rise to various human diseases, including cancer. However, the underlying mechanisms that mediate such precise interactions are not fully explored and the current challenge is to understand how gene regulatory processes are correctly recruited to the genome.
We aim to cover this gap by a) dissecting how interactions between transcriptional or chromatin regulators and the genome are specified, and b) how these interactions impact chromatin and transcription during biological processes. Towards this we combine various experimental and computational strategies, including genome and epigenome engineering, genome-wide studies, proteomics, single-cell measurements and computational modelling. We use mouse embryonic stem cells and their differentiation to neuronal cells as our main model system. This integrative approach allows us to understand gene regulatory mechanisms in a quantitative and functional manner.
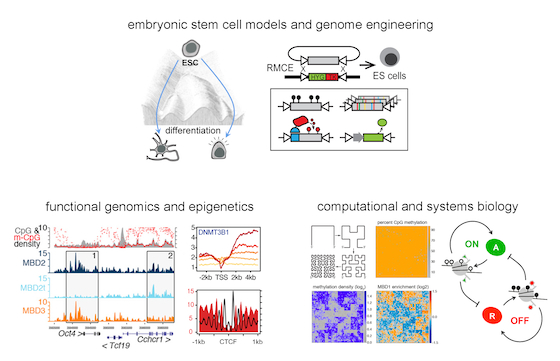
Current topics in the lab are:
Principles and design of epigenetic gene regulatory circuits
DNA sequence-specific transcription factors (TFs) are the key factors that initiate and regulate cell type-specific gene regulation. In addition, various degrees of compaction and chemical modifications of the genome have been identified to provide additional layers of regulation. Correct distribution and interpretation of such “epigenetic” modifications along the genome are highly relevant for gene regulation.
We aim to understand how epigenetic marks are specified and interpreted in a dynamic manner. One major focus is to dissect the mechanisms that guide the specificity of writers, erasers and readers of epigenetic marks to the genome. Based on genome-wide comparisons of numerous epigenetic marks, DNA sequence and transcriptional activity, several correlations and anticorrelations are apparent which suggest a context-dependent crosstalk between these features. Through applying high-throughput functional analysis and reverse engineering of epigenetic regulatory circuits, we want to dissect the underlying principles that mediate context-dependent regulation in vivo, and apply this knowledge to synthetically regulate the chromatin and transcriptional output of a cell.
Function and regulation of DNA methylation in healthy and diseased cells
DNA methylation is the paradigm epigenetic modification associated with transcriptional repression and required for genome function. However, the mechanisms that specify deposition and readout of this mark have not been fully understood in vivo. We have previously explored this by studying genome-wide targeting of the writers and readers of this mark (Baubec et al. Cell 2013 and Baubec et al. Nature 2015). These studies have identified how local context, such as DNA sequence composition, nucleosome positioning or gene activity can influence targeting of these factors to the genome. This knowledge allowed us to predict their binding in different cell types, and furthermore uncovered how mutations in these factors, including the ones identified in ICF or Rett Syndrome patients, result in improper establishment or readout of this relevant epigenetic mark. We are following up on these results to understand how DNA methylation influences tissue-specific transcriptional output in healthy and diseased cells.