Dr. Paul O. Hassa
Research interests
Functional characterization of protein mono-ADP-ribosylation and cross-talk among intracellular mono-ADP-ribosyltransferases in cytokine-mediated signaling pathways in innate immunity and tumorigenesis
The regulation of immune and inflammatory responses is a complex physiological process that is of profound importance to both homeostasis and ultimate survival of an organism. Without inflammation and activation of the immune system an organism could not survive the insult of injury or would rapidly succumb to invading pathogens. The inflammatory response is composed of an elaborate cascade of inflammatory mediators. Their regulation must be tightly coordinated to maintain appropriate and timely immune reactions without an overreaction that can cause damage to the host. Pro- and anti-inflammatory signaling networks must be activated and balanced for an organism to survive in the face of environmental stimuli that elicit an immune response. One of the most dynamic ways to regulate signaling processes is through post-translational modifications.
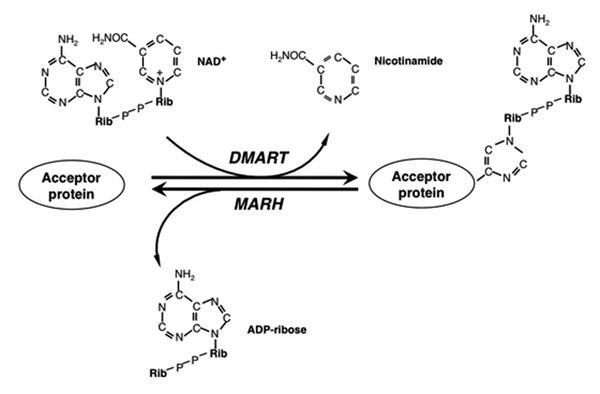
Among these, mono-ADP-ribosylation is one of the first characterized but least understood post-translational modification of proteins. Mono-ADP-ribosylation of proteins is a phylogenetically ancient post-translational modification in which the ADP-ribose moiety of NAD+ is transferred to a specific amino acid of an acceptor protein with the simultaneous release of nicotinamide. The extent of mono-ADP-ribosylation depends on the activity of cellular mono-ADP-ribosyltransferases (mARTs) and mono-ADP-ribose-protein hydrolases (mARHs) that antagonize each other. Mono-ADP-ribosylation has been suggested to be involved in a wide range of physiological and pathophysiological processes (1). Several studies provided preliminary evidence that mono-ADP-ribosylation could play crucial roles in innate immunity. However their exact functions and molecular mechanisms are still elusive. The dissection of mono-ADP-ribosylation networks is central to understand how protein-mono-ADP-ribosylation cycles works in innate immunity. It has been recently demonstrated that several, previously identified intracellular mammalian Diphtheria toxin-related proteins could function as “bona fide” mono-ADP-ribosyltransferases (2). This novel mono-ADP-ribosyl transferase family consists of at least 11 members. Earlier studies provided preliminary evidence that mono-ADP-ribosylation might play crucial roles in innate immunity and tumorigenesis. Recent data suggest that at least 6 members of this novel family of mammalian Diphtheria toxin-related mono-ADP-ribosyl transferases are involved in signaling processes related to innate immunity and tumorigenesis. However the their exact functional roles and molecular mechanisms of endogenous mono-ADP-ribosylation reactions are still elusive and require thorough studies using new strategies and technologies.
Goals
Our goal is to identify and
functionally characterize endogenous protein mono-ADP-ribosylation by
intracellular mono-ADP-ribosyltransferases in cytokine induced signaling
cascades and gene expression with a special focus on
mono-ADP-ribosylation-dependent cross-talk among intracellular
mono-ADP-ribosyltransferases in innate immunity and tumorigenesis. We are also
interested to identify the molecular mechanisms how these epigenetic
mono-ADP-ribose marks on cytoplasmic and nuclear proteins could be recognized
by distinct mono-ADP-ribose-protein binding modules, such as Appr-1/macro
domains. We use a systematic approach, combining genetic, biochemical and
proteomics methods for studying mono-ADP-ribosylation dependent signaling and
gene expression in vivo. Our research projects involve several collaborating
labs in the EU and USA.